India, April 21, 2020 / ABM Respiratory Care, a medical device company focused on the development and commercialization of airway clearance and ventilation solutions, has developed an innovative ventilator which helps healthcare professionals monitor and program the device from anywhere in the world, in real time. ABM is preparing for fast-track approval of its Tele-Ventilator with regulatory agencies in several countries, including India.
The COVID-19 pandemic has highlighted a shortage of ventilators but also a shortage of trained healthcare professionals to manage the large volume of ventilators when needed. In addition, frequent bedside visits by healthcare professionals to adjust and monitor ventilators increase the possibility of infection thereby pushing an already fragile healthcare system into a further grim situation. ABM’s Tele-Ventilator addresses both issues by enabling healthcare professionals to securely monitor and adjust ventilator settings through their online portal from any location.
“AI enabled infrastructure will see a lot of value in the present scenario where healthcare infrastructure has been overburdened because of an unprecedented demand. This ventilator holds immense value for a country like India where health accessibility is a concern due to lack of skilled healthcare professionals in rural areas,” said Vinay Joshi, ABM CEO
ABM’s Tele-Ventilator leverages new technology which is 25 times faster and creates 50 times less data traffic than traditional web technologies. This enables time-valuable, responsive, consistent telemetry and large-scale secure access to ventilators without a complex configuration which is usually needed in traditional connectivity systems.
“Manufacturers will eventually stand up to the challenge of meeting ventilator demand for the current situation but unlike machines, skilled respiratory staff cannot be cloned at required pace” said Vinay Joshi, ABM CEO. “Our tele-ventilation solution will augment access to trained healthcare professional and increase their reach to save lives not only within hospital network but even in most remote parts of the globe”.
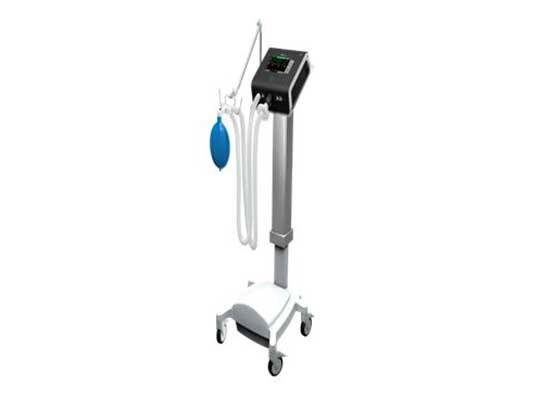
ABM’s Tele-Ventilator, branded as BiWaze™ ION, is based on its BiWaze™ platform developed over the last 30 months and has already US FDA-510(K)-cleared their BiWaze™ Cough system. BiWaze™ ION can be used in both Invasive and Non-Invasive modes, and can be deployed in an ICU, ambulatory or subacute settings for pediatric and adult patients. BiWaze™ ION is a 4 kg, battery-operated, touchscreen-based ventilator which includes advanced ventilator modes as well as key safety and alarm features as per international regulatory standards.
Chad Boerst, Founder and President of ABM Respiratory, said, “Manufacturers around the world are facing difficulty in scaling-up production because of complex mechanical designs which impose huge challenges to secure parts and time to build ventilators. BiWaze™ ION is easy to manufacture and the supply chain is already being streamlined to scale-up production significantly in the coming months”.