Fuel cell technology involves the conversion of the chemical energy of the fuel to electrical energy through a series of chemical reactions. Traditional technologies convert chemical energy from fuel into heat energy before transforming it into a usable form, resulting in reduced efficiency. In contrast, fuel cells directly convert chemical energy into electricity, bypassing the heat production stage and thereby significantly enhancing efficiency.
According to Kings Research, the global fuel cell technology market is anticipated to hit a valuation of $15.35 billion by 2030, growing at a compound annual growth rate of 23.10% from 2023 to 2030. This article explores the industry’s upward trajectory, examining its challenges, and sustainability angle.
What is Fuel Cell Technology?
Fuel cell technology is a clean and efficient method for generating electricity by converting the chemical energy of a fuel, typically hydrogen, directly into electrical energy through an electrochemical process. Unlike batteries, fuel cells do not deplete or require recharging as long as fuel is continuously supplied.
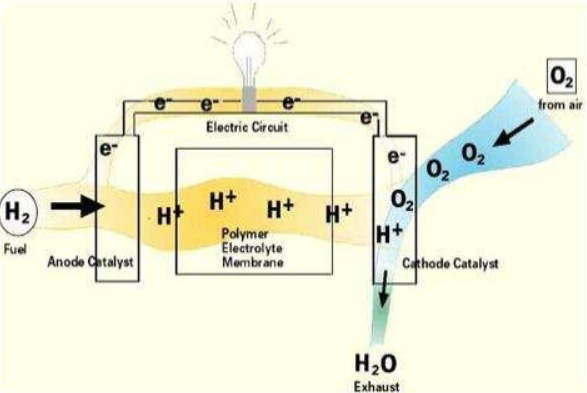
These cells consist of two electrodes—anode (negative electrode) and cathode (positive electrode)—with an electrolyte separating the both. The most common type is the proton exchange membrane fuel cell (PEMFC), which utilizes a polymer electrolyte membrane. Other varieties include molten carbonate fuel cells (MCFC), solid oxide fuel cells (SOFC), and phosphoric acid fuel cells (PAFC).
Why Do Fuel Cells Matter?
Fuel cells present a versatile and sustainable energy solution, applicable across a wide range of sectors, including transportation, industrial, commercial, and residential buildings, as well as long-term energy storage for the grid through reversible systems.
Fuel cells present several advantages over traditional combustion-based technologies used in power plants and vehicles. They operate at higher efficiencies, directly converting chemical energy from fuel into electrical energy, with potential efficiencies exceeding 60%. This direct conversion process leads to minimal or zero emissions compared to combustion engines. For example, hydrogen fuel cells emit only water, positioning them as a crucial solution for tackling climate challenges by eliminating carbon dioxide emissions and other air pollutants that contribute to smog and health issues.
Additionally, fuel cells operate quietly due to their minimal moving parts, further enhancing their suitability for various applications. As the world shifts toward cleaner and more efficient energy solutions, studying and advancing fuel cell technology becomes increasingly important for a sustainable future.
How Does Fuel Cell Technology Work?
Fuel cell technology works by converting the chemical energy of a fuel, typically hydrogen, into electrical energy through an electrochemical process. Here’s a step-by-step explanation of how fuel cells work:
- Fuel Supply: The fuel cell is supplied with fuel, usually hydrogen, at the anode (negative electrode).
- Oxygen Supply: Oxygen, typically from the air, is supplied to the cathode (positive electrode).
- Electrolyte: The anode and cathode are separated by an electrolyte, which allows ions to pass through but prevents the mixing of fuel and oxygen.
- Electrochemical Reaction: At the anode, a catalyst splits hydrogen molecules into protons (H+) and electrons (e-). The protons reach the cathode through an electrolyte, wherein the electrons travel through an external circuit, generating an electric current.
- Electrical Power: The electrical energy produced by the fuel cell can be used to power various devices or systems.
- Reaction at the Cathode: At the cathode, the protons, electrons, and oxygen combine to form water or other byproducts, depending on the type of fuel cell.
How are Governmental Bodies Tackling the Challenges Faced by Fuel Cell Technology?
The U.S. Department of Energy (DOE) is actively collaborating with national laboratories, universities, and industry partners to address critical technical barriers in fuel cell development. The primary challenges in the fuel cell industry are cost, performance, and durability.
- Cost
Research, development, and demonstration (RD&D) efforts are centered on developing low-cost fuel cell stack and balance of plant (BOP) components, as well as advanced high-volume manufacturing approaches to reduce overall system costs. One of the major cost drivers is platinum, a key component of direct hydrogen-fueled polymer electrolyte membrane fuel cells.
Therefore, research is centered on enhancing the activity and efficiency of platinum, minimizing the use of PGM-alloy catalysts and platinum group metals (PGM) and investigating PGM-free catalyst alternatives for sustainable, long-term applications. Some of the alternatives being studied include Ion-Exchange Membrane Electrolytes, Membrane Electrode Assemblies (MEAs), Solid Oxide Fuel Cell (SOFC) Materials, and more.
- Performance
To improve fuel cell efficiency and performance, RD&D focuses on innovative materials and integration techniques. Efforts involve developing ion-exchange membrane electrolytes that offer better efficiency and durability at a lower cost. Additionally, there is an emphasis on enhancing membrane electrode assemblies (MEAs) to achieve high power density by integrating MEA components more effectively.
Modeling is also crucial to understanding system design and operating conditions. The development of stacks with high efficiency at rated power and high-performing BOP components, such as air management systems with low parasitic losses, is another key area of focus.
- Durability
Fuel cell applications require sustained performance over extended periods. The DOE has set ambitious lifetime targets for fuel cell systems: 8,000 hours for light-duty vehicles, 30,000 hours for heavy-duty trucks, and 80,000 hours for distributed power systems.
These applications demand high reliability and robustness under dynamic and harsh conditions, including starting and stopping, freezing and thawing, fuel and air impurities, and varying humidity and load cycles. RD&D focuses on identifying and understanding degradation mechanisms and developing materials and strategies to mitigate their effects, ensuring the chemical and mechanical stability of fuel cell system materials and components.
Is Fuel Cell Technology a Futuristic Solution for Sustainability?
Fuel cell technology holds great promise for a sustainable future as an alternative energy source. This clean energy solution can significantly reduce greenhouse gas emissions and dependence on fossil fuels, addressing critical climate challenges. Ongoing research and development efforts focus on improving fuel cell materials, reducing costs, and expanding infrastructure to accelerate the adoption of this technology. With advancements in materials, integration strategies, and the growing interest in hydrogen as a fuel source, fuel cells are poised to play a significant role in shaping a sustainable future.
Bottom Line
Fuel cell technology has the potential to revolutionize various sectors, from transportation to power generation with its high efficiency, low emissions, and versatility. Ongoing research and development efforts to improve materials, reduce costs, and expand infrastructure are paving the way for the wider adoption of fuel cells, bringing us closer to a sustainable and energy-efficient future.
















